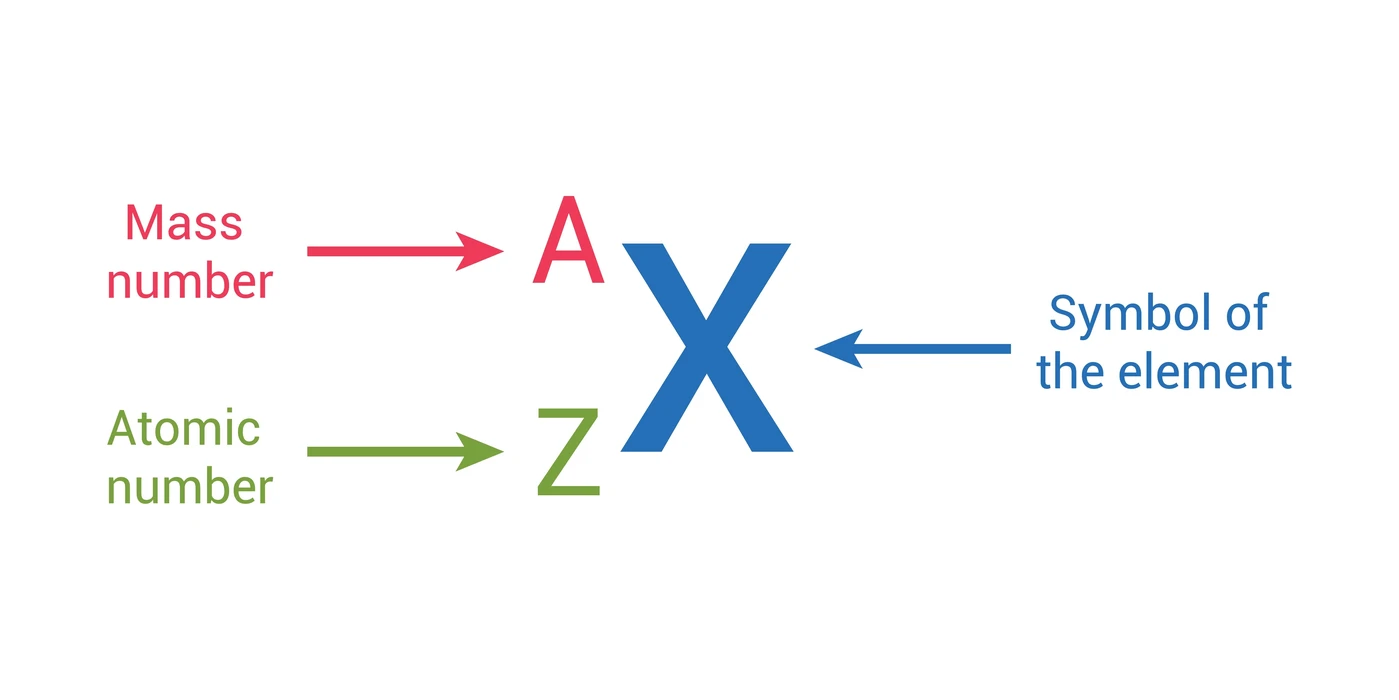Difference Between Atomic Number and Atomic Mass
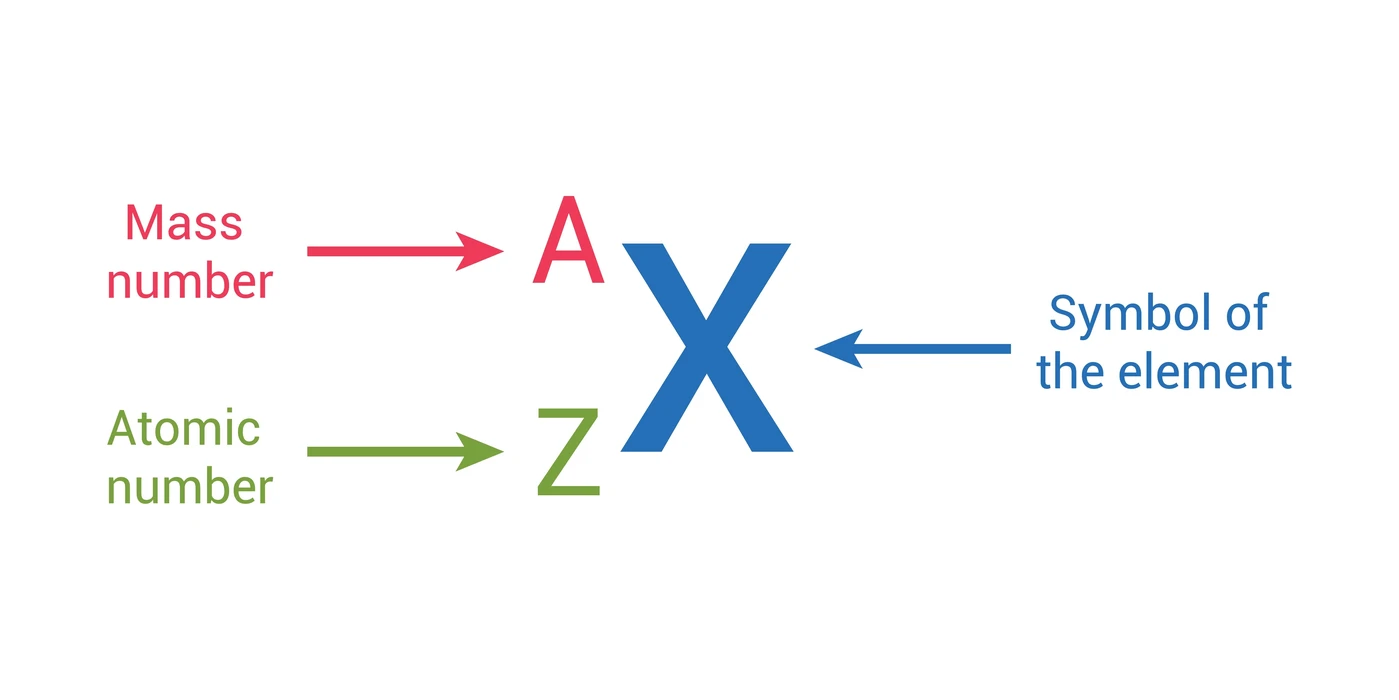
The atomic number and atomic mass are two fundamental properties of atoms that provide important information about their identity and characteristics. Here's the difference between atomic number and atomic mass:
-
Atomic Number:
- Definition: The atomic number (Z) of an atom represents the number of protons found in the nucleus of that atom.
- Symbol: The atomic number is typically represented by the symbol "Z."
- Identifies the Element: The atomic number uniquely identifies the element, as each element has a unique number of protons in its atoms. For example, all hydrogen atoms have an atomic number of 1, all helium atoms have an atomic number of 2, and so on.
- Determines Chemical Properties: The atomic number determines the chemical properties of an element because it defines the number of protons, which in turn determines the element's electronic configuration and how it interacts chemically with other elements.
- Whole Number: The atomic number is always a whole number and cannot be fraction or negative.
-
Atomic Mass:
- Definition: The atomic mass (or atomic weight) of an atom represents the average mass of an atom of that element, taking into account the masses of its isotopes and their relative abundances.
- Symbol: The atomic mass is typically represented by the symbol "A."
- Unit: The atomic mass is usually expressed in atomic mass units (amu) or unified atomic mass units (u), where 1 atomic mass unit is defined as one twelfth of the mass of a carbon-12 atom.
- Not Always Whole Number: The atomic mass of an element is not always a whole number because it is an average of the masses of all the naturally occurring isotopes of that element, weighted by their relative abundances. As a result, atomic masses may be fractional.
- Not Unique to an Element: Unlike atomic number, which uniquely identifies an element, atomic mass is not unique to an element and may vary slightly between different samples of the same element due to variations in isotopic composition.
- Determined Experimentally: Atomic masses are determined experimentally using techniques such as mass spectrometry and are listed in the periodic table as average atomic masses.
In summary, the atomic number of an atom represents the number of protons in its nucleus and uniquely identifies the element, while the atomic mass represents the average mass of an atom of that element, taking into account the masses of its isotopes and their relative abundances.
Thank you,